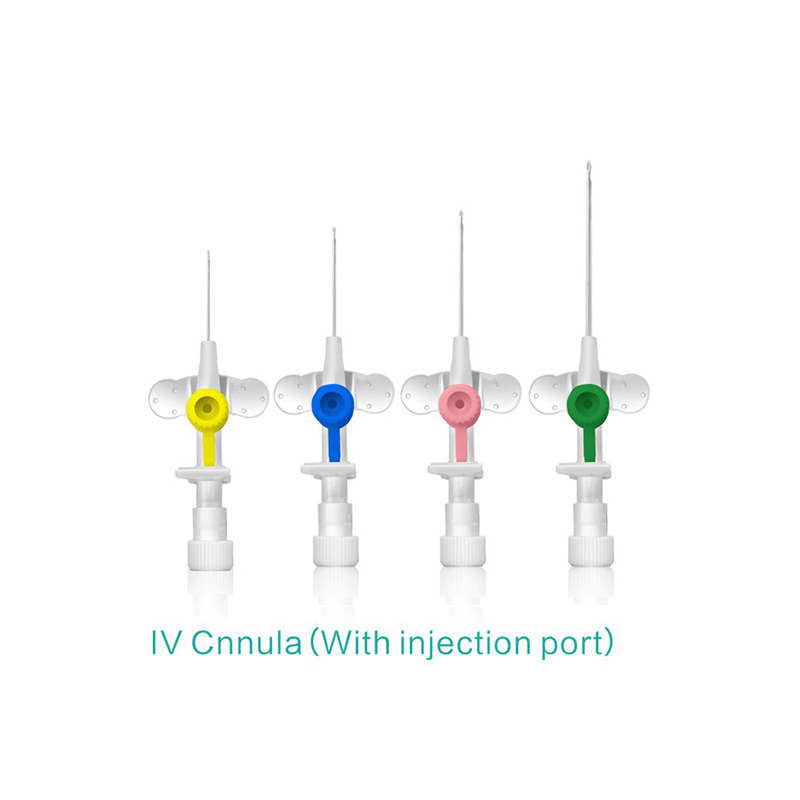
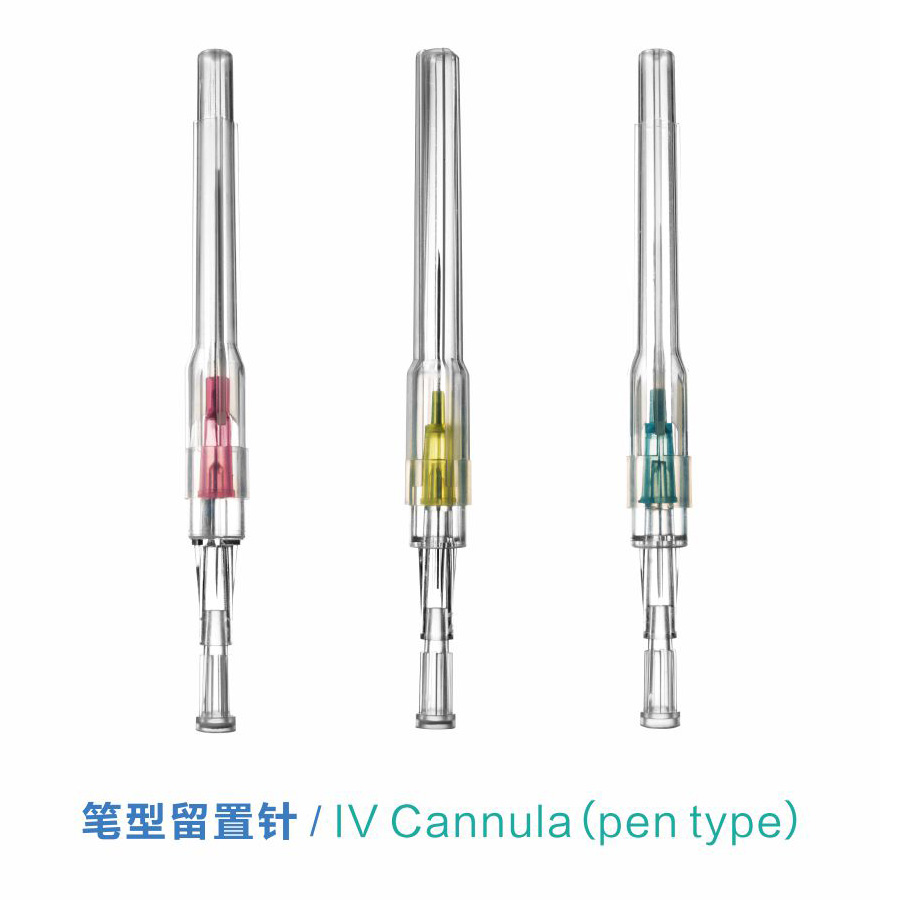
OEM Disposable IV Cannula Supplier for Quality Medical Solutions
When I think about the essential tools for medical procedures, the disposable IV cannula stands out for its reliability and safety. Designed for one-time use, these cannulas help minimize the risk of infection, ensuring peace of mind for healthcare professionals and patients alike. As an OEM supplier, we pride ourselves on delivering high-quality products that meet rigorous medical standards. I understand that choosing the right supplier is crucial for your business, and that's why we focus on innovation and quality control. Our disposable IV cannulas are engineered for easy insertion and patient comfort, helping streamline your operations in any healthcare setting. When reliability matters, I trust our products to exceed expectations. We're committed to being your go-to source for disposable IV supplies, allowing you to focus on what you do best—providing top-notch patient care. Let's connect and make healthcare safer, one cannula at a time.
disposable IV cannula Winning in 2025 From Concept to Delivery
In an ever-evolving healthcare landscape, the demand for innovative medical devices is at an all-time high. One area poised for transformative growth by 2025 is the disposable IV cannula market. With an aim to elevate patient care while minimizing risks, manufacturers are increasingly focusing on developing advanced designs that enhance functionality and safety. These innovations aren't merely conceptual; they are backed by rigorous research and engineering, ensuring that they meet stringent regulatory standards and market expectations. The journey from concept to delivery involves collaboration among various stakeholders, including engineers, healthcare professionals, and regulatory bodies. By fostering partnerships and focusing on user-centered design principles, manufacturers can create solutions that address real-world challenges faced by clinicians. This not only improves patient outcomes but also streamlines operational efficiency in healthcare settings. The integration of smart technology, such as embedded sensors for real-time monitoring, is set to redefine IV therapy, making it safer and more efficient. As we look towards 2025, industry players must remain agile, adapting to shifts in market demands and regulatory changes. Emphasizing sustainability and cost-effectiveness while prioritizing patient safety will be crucial for capturing global procurement opportunities. The future of disposable IV cannulas is not just about the product itself; it's about delivering comprehensive solutions that enhance the overall healthcare experience.
Disposable IV Cannula Winning in 2025: From Concept to Delivery
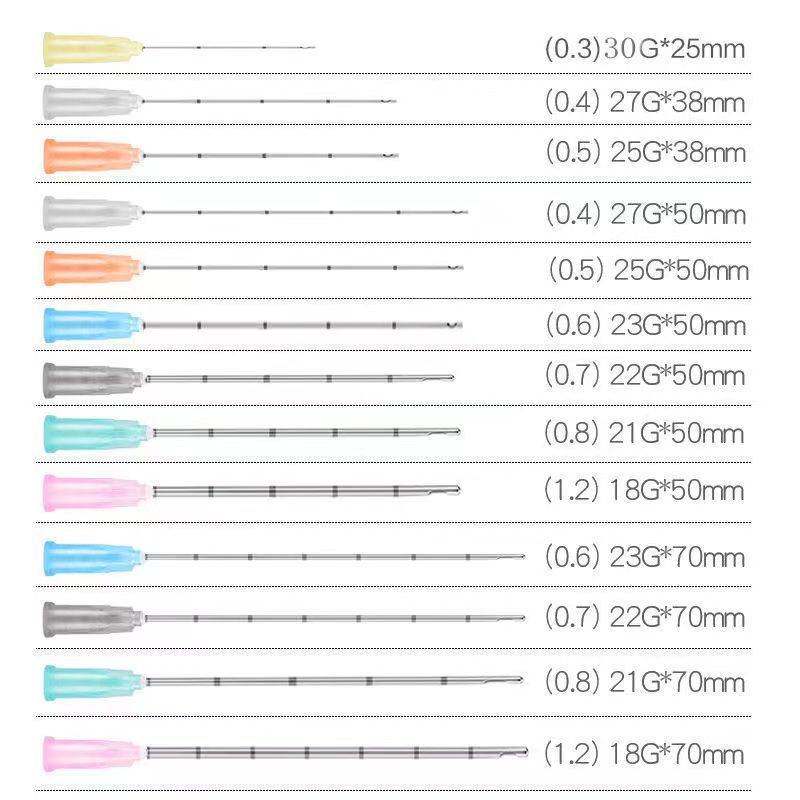
| Dimension | Value |
|---|---|
| Material | Medical-grade Polypropylene |
| Gauge Size | 14G, 16G, 18G, 20G, 22G, 24G |
| Length | 32mm to 80mm |
| Sterilization Method | Gamma Radiation |
| Color Coding | Universal Color System (e.g. Green for 18G) |
| Needle Type | Bevel-edged, Triple-faceted |
| Compliance Standard | ISO 13485 |
| Expected Market Growth | 10% CAGR by 2025 |
Related Products