Vescular Access Products
Vascular access products are used to establish and maintain access to the bloodstream for various medical purposes. They are commonly used for:
Administration of medications and fluids.
Blood sampling.
Hemodialysis.
Parenteral nutrition.
Chemotherapy and other infusion therapies.
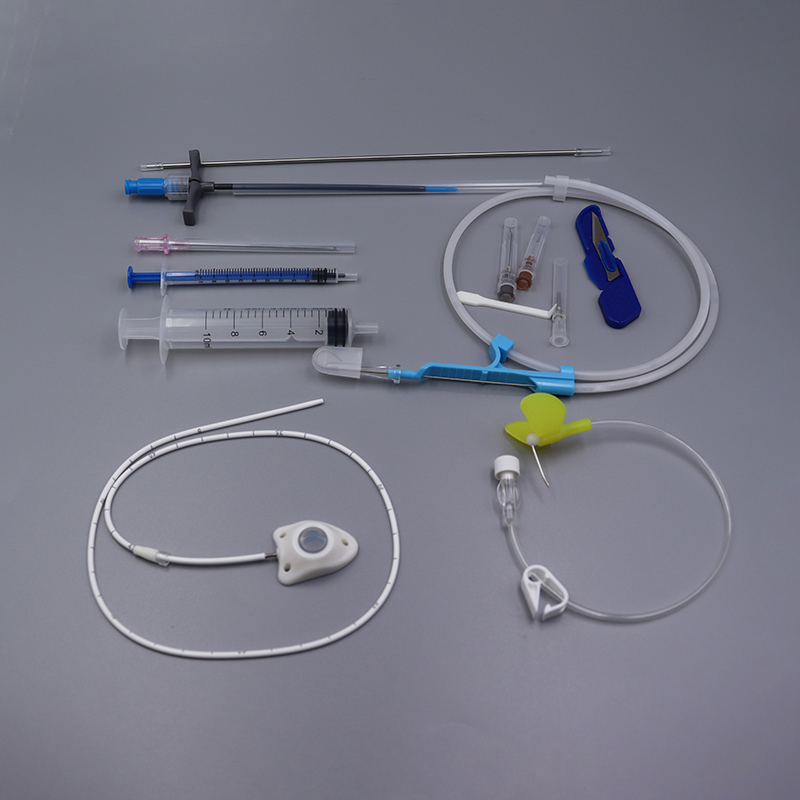
Implantable Port Kit
· Easy to implant. Easy to maintain.
· Intended to reduce complication rates.
· MR Conditional up to 3-Tesla.
· Radiopaque CT marking embedded into port septum for visibility under x-ray.
· Allows for power injections up to 5mL/sec and 300psi pressure rating.
· Compatible with all power needles.
· Radiopaque CT marking embedded into port septum for visibility under x-ray.
Implantable Port – A Reliable Access For Medium And Long-Term Drug Infusion
Implantable Port is suitable for guided chemotherapy for a variety of malignant tumors, prophylactic chemotherapy after tumor resection and other lesions requiring long-term local administration.
Application:
infusion medications, chemotherapy infusion, parenteral nutrition, blood sampling, power injection of contrast.
High safety: Avoid repeated puncture; reduce the risk of infection; reduce complications.
Excellent Comfort: Fully implanted, privacy protected; improve life quality; easy access to medication.
Cost-effective: Treatment period over 6 months; reduce health care cost; easy maintenance, reused for up to 20 years.
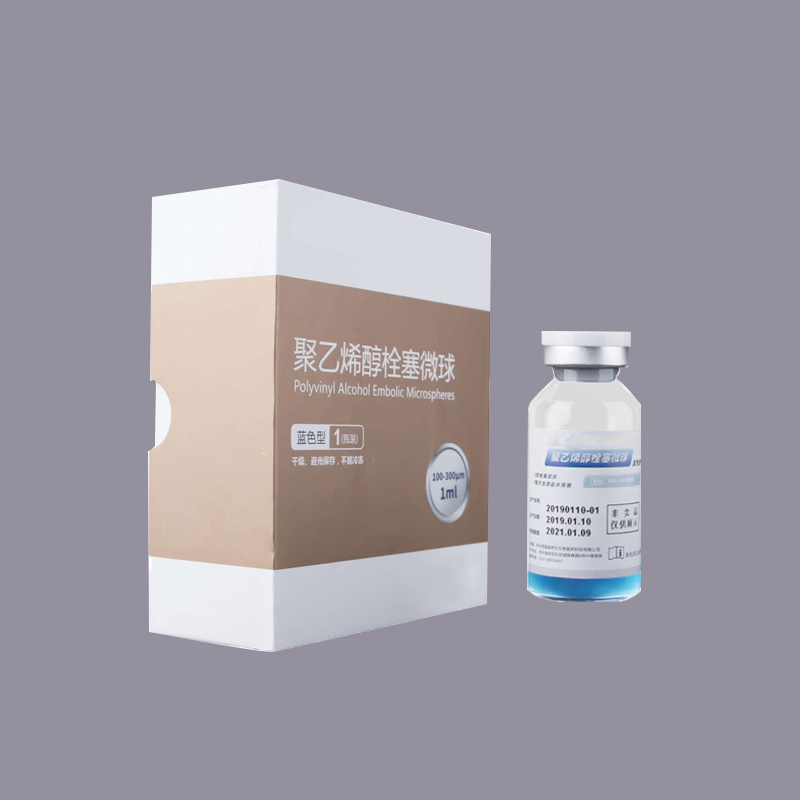
Embolic Microspheres
· Spherical design and conform to blood vessels
· Accurate and long-lasting embolization
· Variable elasticity
· Non-occlusive to microcatheters
· Non-degradable
· Multiple range of specifications and sizes
What is Embolic Microspheres?
Embolic Microspheres are intended to be used for the embolization of arteriovenous malformations (AVMs) and hypervascular tumors, including uterine fibroids.
Embolic Microspheres are compressible hydrogel microspheres with a regular shape, smooth surface, and calibrated size, which are formed as a result of chemical modification on polyvinyl alcohol (PVA) materials. Embolic Microspheres consist of a macromer derived from polyvinyl alcohol (PVA), and are hydrophilic, non-resorbable, and are available in a range of sizes. The preservation solution is 0.9% sodium chloride solution. The water content of fully polymerized microsphere is 91% ~ 94%. Microspheres can tolerate compression of 30%.
Goods preparation
It is necessary to prepare 1 20ml syringe, 2 10ml syringes, 3 1ml or 2ml syringes, three-way, surgical scissors, sterile cup, chemotherapy drugs, embolic microspheres, contrast media, and water for injection.
Step 3: Load the Chemotherapeutic drugs into Embolic Microspheres
Use the 3 ways stopcock to connect the syringe with the embolic microsphere and the syringe with the chemotherapy drug, pay attention to the connection firmly and the flow direction.
Push the chemotherapy drug syringe with one hand, and pull the syringe containing embolic microspheres with the other hand. Finally, the chemotherapy drug and microsphere are mixed in a 20ml syringe, shake the syringe well, and leave it for 30 minutes, shake it every 5 minutes during the period.
Step 1: Configure chemotherapy drugs
Use surgical scissors to uncork the chemotherapeutic medicine bottle and pour the chemotherapeutic medicine into a sterile cup.
The type and dosage of chemotherapeutic drugs depend on clinical needs.
Use water for injection to dissolve chemotherapy drugs, and the recommended concentration is more than 20mg/ml.
After the chemotherapeutic drug was fully dissolved, the chemotherapeutic drug solution was extracted with a 10ml syringe.
Step 4: Add contrast media
After the microspheres were loaded with chemotherapeutic drugs for 30 minutes, the volume of the solution was calculated.
Add 1-1.2 times the volume of contrast agent through the three way stopcock, shake well and let stand for 5 minutes.
Step 2: Extraction of drug-carrying embolic microspheres
The embolized microspheres were fully shaken, inserted into a syringe needle to balance the pressure in the bottle, and extract the solution and microspheres from the cillin bottle with a 20ml syringe.
Let the syringe stand for 2-3 minutes, and after the microspheres settle, the supernatant is pushed out of the solution.
Step 5: Microspheres are used in the TACE process
Through the three way stopcock, inject about 1ml of microspheres into the 1ml syringe.
The microspheres were injected into the microcatheter by pulsed injection.
Prefilled Syringe
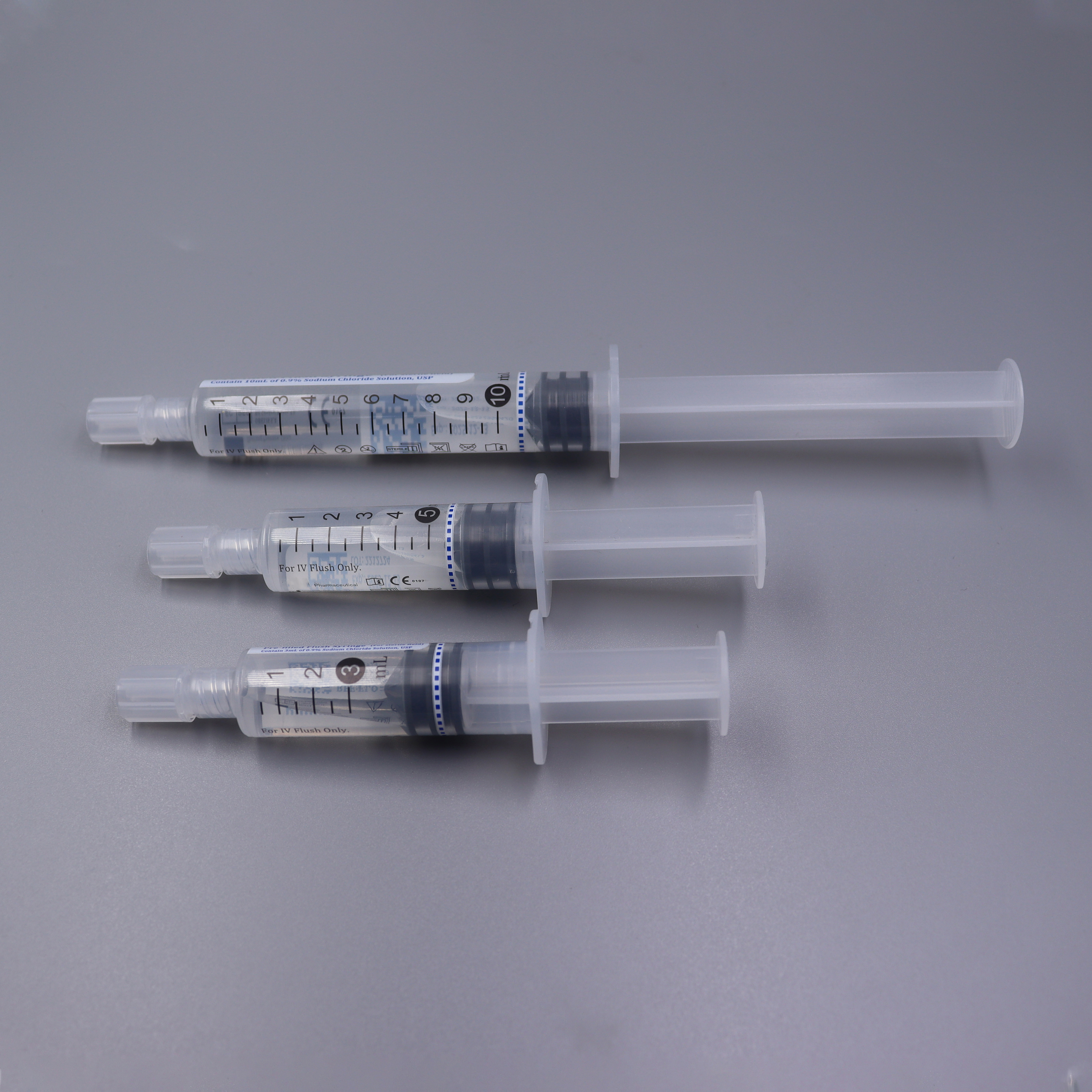
> Disposable Sterile Saline Flush Syringes PP Prefilled Syringe 3ml 5ml 10ml
Structure: The product consists of a barrel plunger piston protective cap and a certain amount of 0.9% sodium chloride injection.
· Fully US cleared.
· No-Reflux technique design to eliminate risk of catheter blockage.
· Terminal sterilization with fluid pathway for safety administration.
· External sterilized flush syringe available for sterile field application.
· Latex-, DEHP-, PVC-Free & Non-Pyrogenic, Non-Toxic.
· Complies with PICC and INS standards.
· Easy screw-on tip cap to minimize microbial contamination.
· Integrated needle-free system maintains the patency of indwelling intravenous access.
Disposable Huber Needle

· Special needle tip design to prevent rubber fragment contamination.
· Luer connector, equipped with needleless connector.
· Chassis sponge design for a more comfortable application.
· Can be equipped with needleless connector, heparin cap, Y three-way
EN ISO 13485 : 2016/AC:2016 Medical equipment quality management system for regulatory requirements
EN ISO 14971 : 2012 Medical devices - Application of risk management to medical devices
ISO 11135:2014 Medical device Sterilization of ethylene oxide Confirmation and general control
ISO 6009:2016 Disposable sterile injection needles Identify color code
ISO 7864:2016 Disposable sterile injection needles
ISO 9626:2016 Stainless steel needle tubes for the manufacture of medical devices
Safety Huber Needle

· Needle-stick prevention, safety assured.
· Special needle tip design to prevent rubber fragment contamination.
· Luer connector, equipped with needleless connector.
· Chassis sponge design for a more comfortable application.
· High pressure resistant central line with 325 P.S.I
· Y port optional.
EN ISO 13485 : 2016/AC:2016 Medical equipment quality management system for regulatory requirements
EN ISO 14971 : 2012 Medical devices – Application of risk management to medical devices
ISO 11135:2014 Medical device Sterilization of ethylene oxide Confirmation and general control
ISO 6009:2016 Disposable sterile injection needles Identify color code
ISO 7864:2016 Disposable sterile injection needles
ISO 9626:2016 Stainless steel needle tubes for the manufacture of medical devices
We Have More Than 20+ Years Practical Experience in Industry
With over 20 years of healthcare supply experience, we offer a wide product selection, competitive pricing, exceptional OEM services, and reliable on-time deliveries. We have been the supplier of the Australian Government Department of Health (AGDH) and the California Department of Public Health (CDPH). In China, we rank among the top providers of Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle and Paracentesis products.
By 2023, we had successfully delivered products to customers in 120+ countries, including the USA, EU, Middle East, and Southeast Asia. Our daily actions demonstrate our dedication and responsiveness to customer needs, making us the trusted and integrated business partner of choice.

Factory Tour

Our Advantage

Highest quality
Quality is the most important requirement for medical products. To ensure only the highest quality products, we work with the most qualified factories. Most of our products have CE, FDA certification, we guarantee your satisfaction on our entire product line.

Excellent Service
We offer complete support from the beginning. Not only do we offer a wide variety of products for different demands, but our professional team can assist in personalized medical solutions. Our bottom line is to provide customer satisfaction.

Competitive pricing
Our goal is to achieve long-term cooperation. This is accomplished not only through quality products, but also striving to provide the best pricing to our customers.

Responsiveness
We are eager to help you with whatever you may be looking for. Our response time is quick, so feel free to contact us today with any questions. We look forward to serving you.
Support & FAQ
A1: We have 10 years experience in this field,Our company has professional team and professional production line.
A2. Our products with high quality and competitive price.
A3.Usually is 10000pcs; we would like to cooperate with you, no worries about MOQ, justsend us of your what items you want order.
A4.Yes, LOGO customization is accepted.
A5: Normally we keep most of the products in stock, we can ship samples out in 5-10workdays.
A6: We ship by FEDEX.UPS,DHL,EMS or Sea.
Feel Free To Reach Out To Us If You Have Any Questions
We will reply you via emial in 24 hours.


