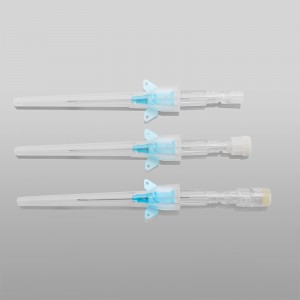
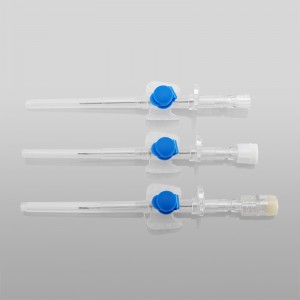
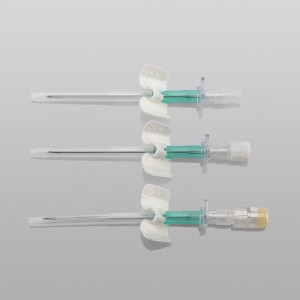
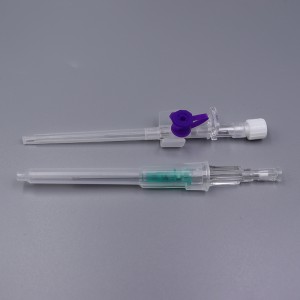
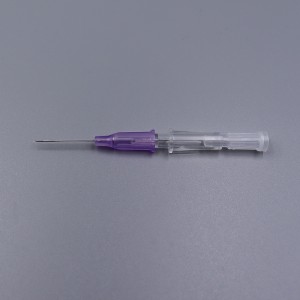
CE ISO FDA Certified Medical Supply Disposable IV Cannula
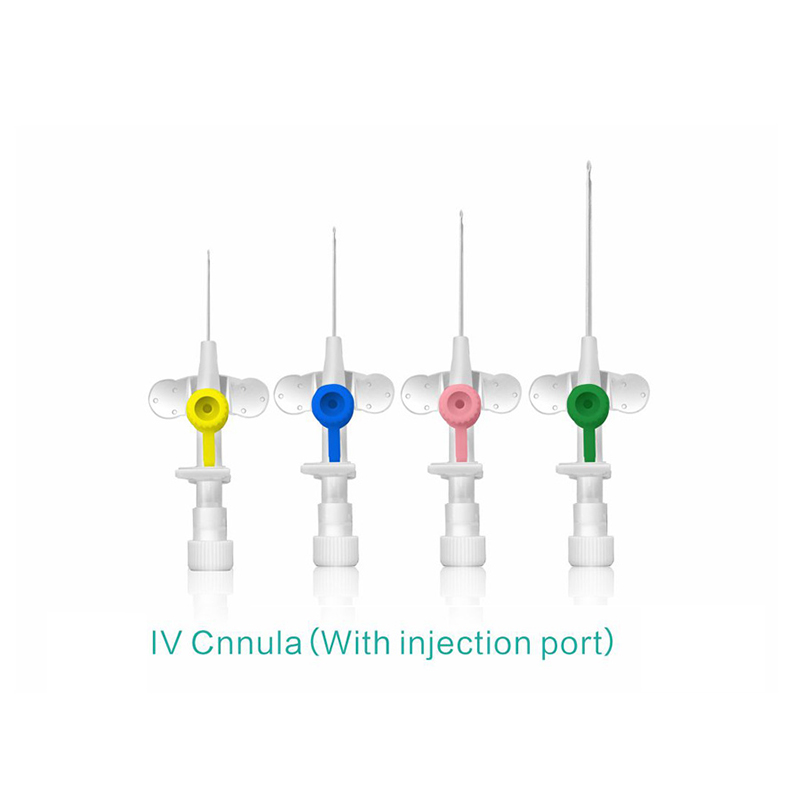
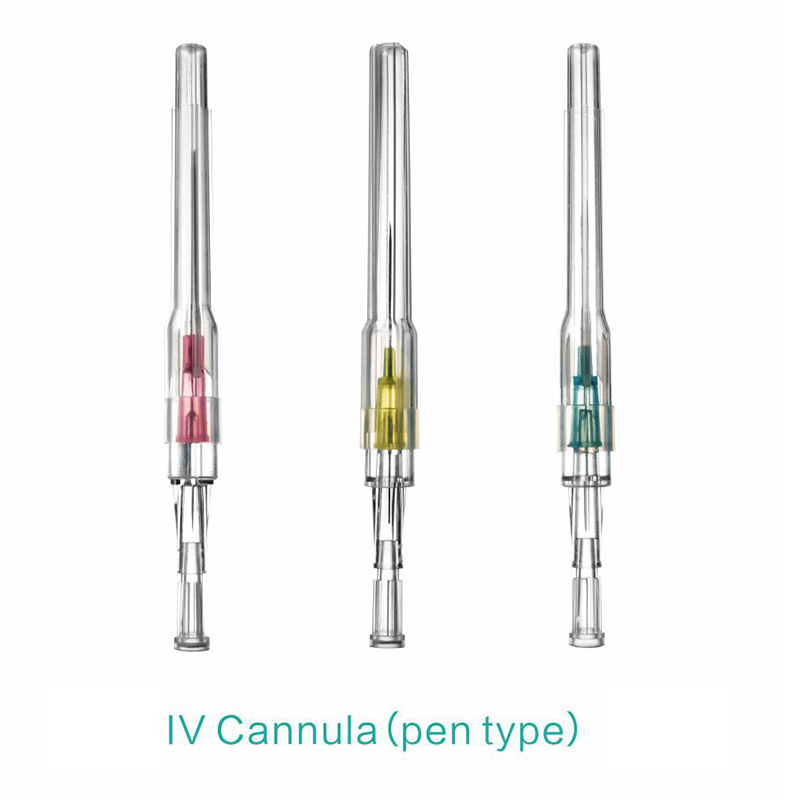
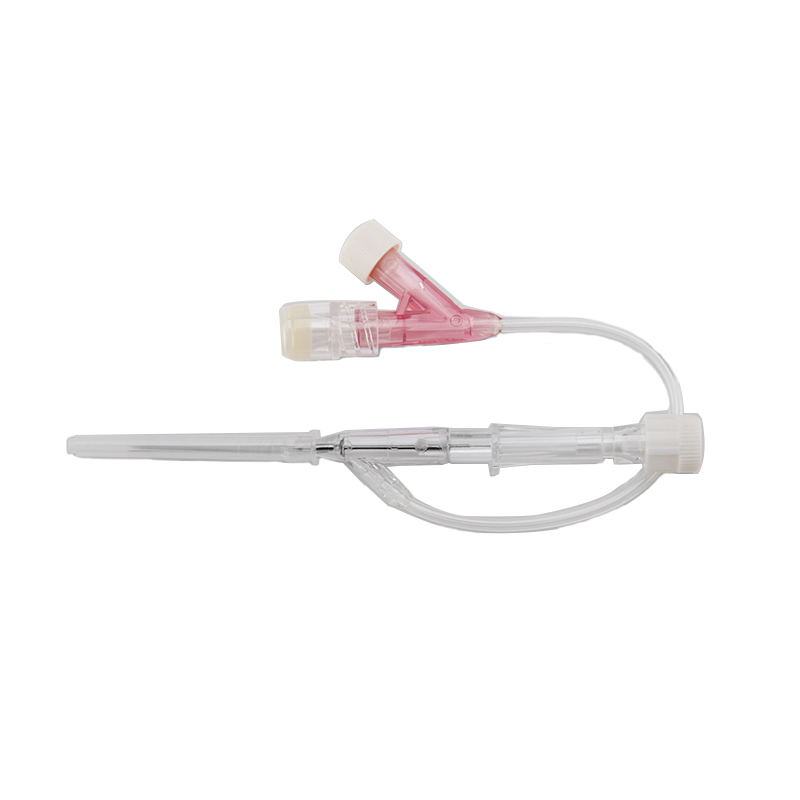
An intravenous (IV) cannula is a medical device used to administer fluids, medications, and blood products directly into a patient's vein.
Specification
Size: 14G,16G,18G, 20G,22G,24G and 26G
Feature
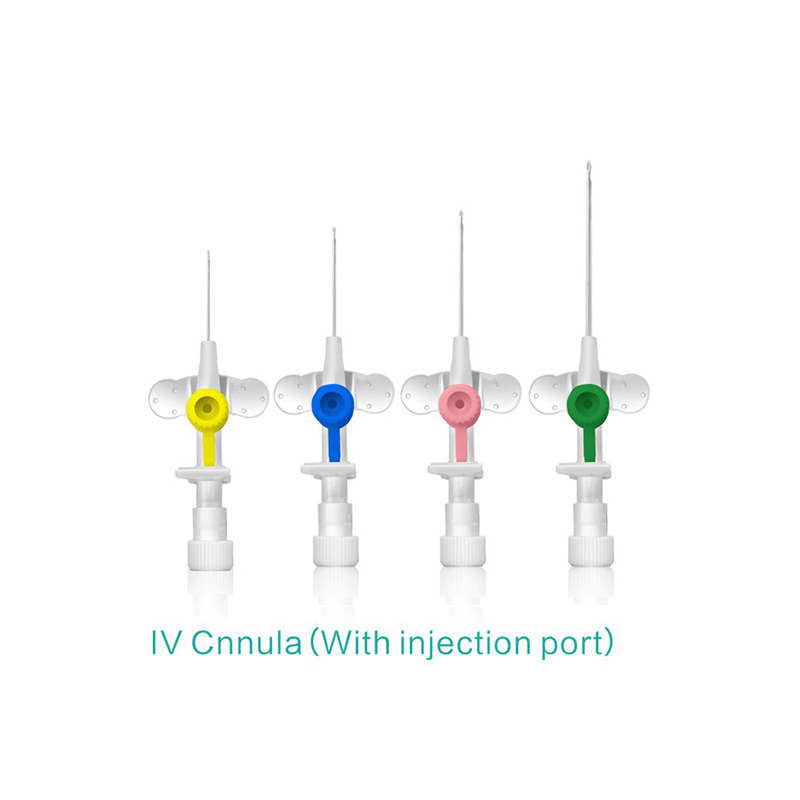
Color-coded casing cap allows for easier identification of catheter size.
Translucent catheter hub and flashback chamber allows for easy detection of blood.
Teflon Radio-opaque catheter.
Precision finished PET or PU catheter assures stable flow and eliminates catheters tip kink.
Can be connected to syringe by removing filter cap to expose lure taper end.
Use of hydrophobic membrane filter eliminates blood leakage.
Close and smooth contact between catheter tip and inner needle enable safe and smooth.
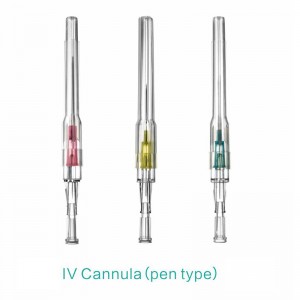
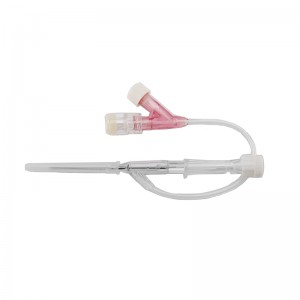
Pen type, winged type, butterfly type, Y type, etc.
Injection port is optional.
CE
ISO13485
USA FDA 510K
EN ISO 13485 : 2016/AC:2016 Medical equipment quality management system for regulatory requirements
EN ISO 14971 : 2012 Medical devices - Application of risk management to medical devices
ISO 11135:2014 Medical device Sterilization of ethylene oxide Confirmation and general control
ISO 6009:2016 Disposable sterile injection needles Identify color code
ISO 7864:2016 Disposable sterile injection needles
ISO 9626:2016 Stainless steel needle tubes for the manufacture of medical devices

SHANGHAI TEAMSTAND CORPORATION is a leading provider of medical products and solutions.
With over 10 years of healthcare supply experience, we offer a wide product selection, competitive pricing, exceptional OEM services, and reliable on-time deliveries. We have been the supplier of the Australian Government Department of Health (AGDH) and the California Department of Public Health (CDPH). In China, we rank among the top providers of Infusion, Injection, Vascular Access, Rehabilitation Equipment, Hemodialysis, Biopsy Needle and Paracentesis products.
By 2023, we had successfully delivered products to customers in 120+ countries, including the USA, EU, Middle East, and Southeast Asia. Our daily actions demonstrate our dedication and responsiveness to customer needs, making us the trusted and integrated business partner of choice.

We have gained good reputation among all of these customers for good service and competitive price.

A1: We have 10 years experience in this field,Our company has professional team and professional production line.
A2. Our products with high quality and competitive price.
A3.Usually is 10000pcs; we would like to cooperate with you, no worries about MOQ, justsend us of your what items you want order.
A4.Yes, LOGO customization is accepted.
A5: Normally we keep most of the products in stock, we can ship samples out in 5-10workdays.
A6: We ship by FEDEX.UPS,DHL,EMS or Sea.